Winter
Diet Composition of Introduced Rainbow Trout and Macroinvertebrate Assemblages
in the Guadalupe River, Texas
Mario
Sullivan
Department of Biology, Aquatic
Station,
Texas State University
San
Marcos, Texas 78666 USA
E-mail:
ms1667@txstate.edu
Archis
Grubh
Inland
Fisheries Program
Texas
Department of Parks and Wildlife
San
Marcos, Texas 78666 USA
Yixin
Zhang
Department
of Biology, Aquatic Station, Texas State University
San Marcos, TX 78666, USA
Timothy
Bonner
Department
of Biology, Aquatic Station, Texas State University
San Marcos, TX 78666, USA
ABSTRACT
Winter diets of
fish are often related to aquatic macroinvertebrate availability in the
habitats. This study assesses the winter diets of introduced rainbow trout (Oncorhynchus mykiss) and body condition in
downstream habitats of Canyon Lake reservoir on the Guadalupe River, a
tailwater system in South Central Texas.
Both benthic and drift samples of macroinvertebrates were taken to
determine where these introduced trout focused their feeding habits. Among 46 trout sampled, diets mostly
consisted of gastropods and vegetative matter such as detritus and leaves (21
and 22% by mass, respectively). This
diet was consistent among size classes (0-249, 250-299, and >300 mm SL) and
trout sampled did not feed upon prey commensurate with their relative
abundances captured in benthic and drift samples. The most abundant macroinvertebrate taxa both
on the benthos and within the drift were Ephemeroptera, Diptera, and Amphipoda
yet these were minor constituents in the diet samples. The results suggest that food availability may
be not a major limiting factor for this fishery. There is evidence to suggest
that these trout may not feed on an optimal diet relative to what is available. Despite the implication that these trout fed
upon a relatively poor diet, most of the trout sampled were in good body
condition, and there is ample wintertime benthic forage to accommodate the
put-and-take trout fishery in the tailrace section of the Guadalupe River.
INTRODUCTION
Tailwater
fisheries represent a very distinct type of lotic habitat in which fish, often
salmonids, are stocked downstream of a major impoundment for angling. Tailwaters
create habitats with cooler thermal regimes than what would otherwise occur. This allows for cold water species, especially
salmonids, to persist in areas they would not normally occur, offering unique
angling opportunities in some systems. In
contrast, because tailwaters are often unproductive and do not offer quality
spawning substrates, growth and reproductive success are highly variable, so
tailwater populations are often supplemented by stocking (Weiland and Hayward
1997; Johnson et al. 2006). Managers of
tailwater fisheries must therefore balance flow regimes to serve both the
aquatic ecosystem (with respect to both game and non-game species) as well as
civil needs such as hydroelectric power and flood control (Jacobs et al.
1987).
In
order to protect the investment various fisheries management agencies put into
tailwater fisheries in terms of stocking and creating access for anglers, an
understanding of the factors that influence growth and reproduction of fishes
in tailwater systems is of importance. Salmonid
abundance and growth are primarily dependent upon food availability (Chappman
1966, Mason 1976; Murphy et al. 1981; Cada et al. 1987, Richardson 1993) as
well as favorable water chemical conditions and suitable instream habitats
(Poff and Huryn 1998, Bettoli et al. 1999).
Salmonids are visual predators that feed on drifting invertebrates in the
water column (Elliott 1973), but switch opportunistically from drift to benthic
feeding in response to habitat conditions and food supply (Nislow et al. 1998,
Zhang and Richardson 2007). Because
invertebrate production represents the transformation of stream’s energy base
to a form readily available for salmonids, information of invertebrate
diversity, abundance, and habitat associations is critical for managing and
sustaining a salmonid fishery. However,
impoundments cause environmental changes that subsequently alter the dynamics
of both fish and aquatic invertebrate community structure and function (Bunn
and Arthington 2002).
Johnson
et al. (2006) used a bioenergetics approach to investigate limiting factors
with respect to the growth of brown trout (Salmo
trutta) in a tail-water fishery. The
authors found that food availability was the major limiting factor in terms of
brown trout growth rates. While certain
prey items were abundant, especially isopods, there was a lack of larger, more
energy rich food items. It was also
noted that if the water temperatures were to increase, it would put a further
metabolic load on the fishes causing an even greater energetic deficit. If this is true, then it is prudent for
managers of any tailwater fishery to have some idea of the available forage and
what is actually being consumed by the fish species of interest even if a full
bioenergetics approach is not practical.
Canyon
Lake Reservoir of the Guadalupe River is a popular and economically important
trout fishery in South-Central Texas as it provides one of the few
opportunities within the state for anglers to catch Salmonids year-around. Texas Department of Parks and Wildlife (TPWD)
estimated that the fishery was worth ca. US$164,537 between 2004 and 2005. Additionally, 90% of the anglers interviewed were
happy with the angling opportunities at the tail-water fishery at Canyon Lake
Reservoir (Bradle et al. 2006). Given the importance of this fishery and the
management issues associated with tailwaters, an understanding of the
relationships between habitat and the overall condition of trout are necessary
to properly manage this system in order to maintain an economic resource.
Recent
studies have addressed rainbow trout survival (Magnelia 2004) and its diet
(Halloran 2000) in the Canyon Reservoir tailwaters. Collectively, these studies found that
rainbow trout (Oncorhynchus mykiss)
survive up to 17 km downstream from Canyon Reservoir tailrace (Magnelia 2004)
and that the most abundant drifting macroinvertebrate taxa from the tailwater were
underrepresented in the trout guts, suggesting that trout are dependent more on
benthic taxa than drifting taxa (Halloran 2000). The
purpose of this study is to determine the seasonal benthic macroinvertebrate
assemblage of this tailwater fishery with respect to habitat, assess the winter
diets of rainbow trout, and determine their body condition. Because tailwater fisheries can be relatively
unproductive to begin with, it is especially important to determine the diets
and body condition of these trout during winter, a time of particularly low
benthic productivity.
METHODS
AND MATERIALS
Canyon
Reservoir was built in 1964 on the Guadalupe River in the southeastern region of
the Edwards Plateau of Texas and is classified as an oligomesotrophic deep water
reservoir (Hannan et al. 1979). Canyon Reservoir
tailrace was first stocked with rainbow trout by Texas Parks and Wildlife
Department in 1966 (White 1968) and has since been a popular put-and-take
winter fishery. During the study period
(August 2006 – July 2007), the mean monthly discharge ranged from a low of 1.5
m3/s (August 2006) to a high of 39.3 m3/s (April 2007)
and the maximum daily discharge was 150.9 m3/s occurring on May
31st, 2007 (USGS Station No. 08167800).
Maximal discharges were recorded during this study period (March through
September), but cycles of such high discharge were periodically observed at a
frequency of every 3 to 5 years, although with increasing intensity in the
recent years. The mean maximum
temperature in the Canyon Reservoir hypolimnion generally occurs during October
(Groeger and Tietjen 1998), but the maximum temperature occurred during August 2006
at Site 4 (21.4 ºC).
This
study was conducted at four sites on the main-stem of the Guadalupe River between
August-06 and July-07 (Figure 1). Site 1
was characterized by 50% riffle with gravel and cobble substrates, and 50% run
with bedrock substrate. Site 2 was a
long run with 50% sandy substrate, and 50% bedrock substrate with deep
longitudinal gullies toward the mid-section of the river. Site 3 was characterized by a long stretch of
riffle or run depending on the water depth, with gravel and cobble substrates
all along. Site 4 had the deepest mean
cross-section with bedrock substrate and several deep longitudinal gullies.
At each site, the following water quality and
environmental parameters were recorded on a monthly basis: temperature (ºC),
conductivity (μS/cm), pH, dissolved oxygen (mg/l), and turbidity (NTU)
using a YSI-Model 600 multi-probe meter.
Mean depth (m) and current velocity (m/s) were obtained at each site using
transects on 3 to 4 cross-sectional profiles.
Water discharge from Canyon Reservoir was obtained from the USGS Gaging
Station on the Guadalupe River at Sattler, TX (Station Number 08167800).
Benthic macroinvertebrates were collected at all four
sites between August 2006 and July 2007 near the 15th of each month with
a D-net from available mesohabitats (i.e., near shore, pools, runs, and
riffles) and one, five-minute Surber sample was taken at each site in runs or
riffles. Drift net samples (mesh size =
250 μm) were conducted during the month of February 2007 at the first
three sites in order to coincide with rainbow trout diet samples. Two drift nets were placed side by side and
oriented in the direction of current at 0900 for 24 hours and nets were emptied
every three hours. All macroinvertebrate
samples were preserved in the field with 95% ethanol and identified in the
laboratory to lowest practical resolution using multiple keys (Peckarsky et al.
1990, Merritt and Cummins 1996, McCafferty 1998, Smith 2001).
Rainbow
trout were sampled using an electro-fishing boat in February 2007 and each site
was electro-fished with two passes. At
time of capture, trout were placed on ice to slow the digestive process. In the laboratory, fish lengths (SL) and weights
(g) were measured. In order to use
relative and standard weight equations based on total length (TL), standard
lengths were converted using Carlander (1970).
Stomachs were preserved in 70% ethanol for diet analyses. Stomach contents from each fish were blotted
dry and weighed to the nearest milligram, sorted and identified with the aid of
a dissecting microscope.
Macroinvertebrates were identified to the lowest practical taxonomic
level and unidentifiable material was listed as detritus matter. All diet analyses are expressed quantitatively
as percent abundance by relative mass (g).
The relative weight (Wr)
of all trout sampled was calculated using the standard weight (Ws) equation from Murphy and Willis
(1996) for lotic rainbow trout; log10 (Ws) = −5.023 – 3.024 ∙ log10 (total length,
mm). Trout standard lengths were
converted to total lengths using the equation in Carlander (1970) where TL = 1.149 (SL). In order to determine
whether or not trout were feeding from the benthos or drift, we used a
chi-squared test (α = 0.05). Expected
values were calculated using the total mass of diet remains across all rainbow
trout stomachs and multiplied by the proportions of the most abundant taxa
collected in the winter benthic samples (taken in December 2006, January 2007,
and February 2007). The resulting
expected values then represent the expected mass in each stomach for taxa that
had ≥ 1.0% relative abundance by number.
Aquatic macroinvertebrate diversity for each site was calculated using
both Shannon-Wiener (H) and Simpson’s
(D) diversity indices. Multivariate direct gradient ordination
technique (canonical correspondence analysis; CCA) was used to explore
relationships among macroinvertebrate abundance, habitat variables, sites and
seasons (ter Braak 1986). Abundances were log10(x + 1) transformed and rare taxa were
down-weighted (McCune and Mefford 1999).
RESULTS
A total of 13,033 macroinvertebrates was collected in
benthic samples. The seasonal patterns
among the most abundant benthic macroinvertebrates (≥ 2% relative
abundance) were variable with high turnover observed among several taxanomic groups
(Figure 2). Benthic macroinvertebrates
with the greatest turnover were Dipterans and Ephemeropterans. During the summer (Figure 2a), the community was more evenly split
between the dominant taxa (Diptera = 30%, Hemiptera = 28%, Ephemeroptera = 14%
and Gastropoda = 13%). As the year
progressed into the cooler seasons, Ephemeropterans became increasingly
abundant, constituting 47% of the total benthic community by winter (Figure 2c).
During the spring, dipterans largely dominated the community, comprising
49% of the benthic community (Figure 2d). In terms of benthic aquatic insect predators,
winter was the only season in which odonates were captured, and they
represented a small proportion of the community (2.2%). The most dominant predaceous taxa among the
benthic samples were Ambrysus spp.
(Hemiptera: Naucoridae). Their abundance
peaked in summer (28%). Trichopteran
relative abundance remained relatively constant throughout the seasons but
peaked in winter (7%) and reached a minimum during the summer (5%).
A total of 797 macroinvertebrates was
collected in the drift net samples and, in general, chironomids (Diptera)
dominated the drift samples (Figure 3). Site
3 had the greatest diversity values and Site 2 had the lowest (Table 1). Drift samples from night had greater genus
richness and diversity values compared to the day samples at site 3 but the
trend was reversed at Sites 1 and 2.
Among all the taxa, Chironomidae had the highest numbers at both day and
night samples. Amphipods and Dytiscids
appeared only during the night samples.
Baetidae (Ephemeroptera), Isonychia
(Ephemeroptera), Simuliidae (Diptera), Stenonema
(Ephemeroptera), and Tricorythidae (Ephemeroptera) had greater abundances at
night than during the day.
Along the first CCA axis, sites are separated
along gradients (loadings shown in parentheses) based on pH (0.76), depth
(0.68), and dissolved oxygen (0.41), Figure 4.
For the variable pH, the measurements did not vary a great deal among
sites (mean = 8.3, sd = 0.15, range = 8.0 – 8.6). Sites 1 and 2 tended to have greater pH
values (mean = 8.3) and Sites 3 and 4 were lower (mean = 8.2), as Site 4
experienced the minimum pH value in July. Sites 1 and 2 had greater DO values as
well as greater mean depths than Site 3.
On the second CCA axis, sites separate along a gradient of velocity and
turbidity. Among these, temperature is
the most important in site ordination (CCA loading = 0.61). Sites 1 and 2 were cooler, experiencing mean
temperatures of 15.0 and 15.4 ºC, respectively and Sites 3 and 4 were warmer,
experiencing mean temperatures of 16.0 and 17.0 ºC, respectively. Turbidity
(CCA loading = 0.41) tended to be lower in Sites 1, 2, and 4 (mean for Sites 1,
3 and 4 = 4.0) but at Site 3 the mean was 5.3.
Velocity was also important (CCA loading = 0.26); Site 3 had the
swiftest flows (0.60 m/s) and Site 2 had the slowest average flows (0.24
m/s). The
macroinvertebrate taxa shown on the bi-plot are those found in the greatest
abundance in rainbow trout diets. Elmidae (Coleoptera) and Hydropsychidae
(Trichoptera) occurred in the regions characterized by greater current
velocities. In contrast, the burrowing
mayfly genus Hexagenia was greater in
abundance at sites 3 and 4 which were characterized by greater maximum depths
and more moderate flows.
Across
sites, rainbow trout in the Guadalupe River tailwaters did not feed in
proportion to available benthic food items (χ² = 29.4, df = 5, p <
0.001, see Table 2 for chi-square test data) during the winter. Algae was the most abundant diet item by
mass (22%) while Gastropoda was second most abundant (21%). Algae were also the most common item by
occurrence (67%). The second most abundant
item was Gastropoda (58%) although Gastropoda only contributed about 7% of the
total abundance in the benthic samples. Terrestrial
food items were not an important diet component in the winter time for these
trout. The only terrestrial food items observed
were formicids (ants) and they contributed < 1% by mass.
The diet composition of the most abundant food items
(≥2% by mass) was variable among sites but some food item categories
remained relatively consistent (Figure 6).
For example, trout at all four sites consumed algae and detritus but
these two diet items were most abundant in diets sampled at Sites 2 and 3. Also, gastropods were observed in diets at
all four sites but diets from Sites 1 and 3 contained the greatest proportions
(20 and 25%, respectively). Trout
captured in Sites 3 and 4 (furthest distance from dam) did not consume
gastropoda to a great extent but primarily consumed detritus and
ephemeropterans. There was also a
greater abundance of unidentified insect remains among these sites. This indicates that trout in these sites
consumed more insects in general, versus the gastropods and plant materials
consumed in Sites 1 and 2. Isopods were
only found in diets from Sites 1 and 2; contributing 37% of the overall diets
sampled at Site 2.
With respect to fish size and diet composition, larger
trout (>300 mm SL, n = 11) consumed more fish but this size class also
tended to consume more algae and detritus (56%), Figure 5. Gastropoda constituted at least 20% by mass of
trout diets across all size classes sampled (28% in 0 – 241, 14% in 253 – 299,
and 10% in 304 – 379 mm SL). In none of
the size classes sampled did individuals fish feed on items in proportion to their
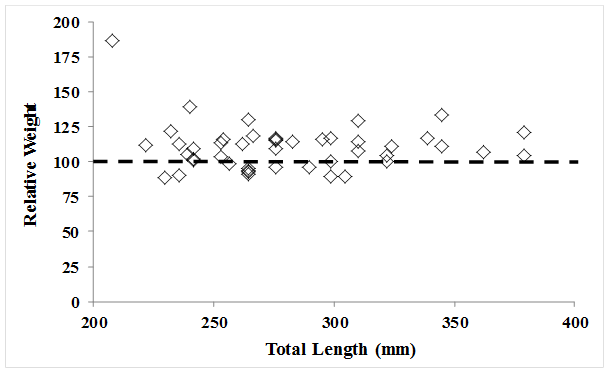
availability. Among the 46 fish sampled,
76% had relative weights (Wr) 100% or
above, and the mean Wr was 110 (Figure
7).
DISCUSSION
Rainbow trout in
the Guadalupe River did not feed in proportion with the relative abundance of
macroinvertebrate availability in the habitats.
This discrepancy in diets among sites may reflect some of the seasonal
associations of habitats. All sites in
the study area contained bedrock substrate but Site 3 was characterized by a
long riffle or run, depending on water depth, which may have provided a more
optimal habitat for benthic macroinvertebrates.
Since February was characterized by low discharges, this was a riffle
complex when diets were sampled, which could explain the greater abundance of
insects in the diets. This is also
supported in Table 1 where Site 3 has the greatest diversity values compared to
Sites 1 and 2 for February invertebrate sampling. In terms of habitat, Site 3 had much more
gravel and cobble than Site 4 which primarily contained bedrock. Isopoda were only present in Sites 1 and 2
(closest to the spillway of the dam) which could indicate an important food
resource for these fishes is coming from the reservoir’s benthos.
Ephemeropterans became more abundant in diets with
increased distance from the reservoir, which suggests increased downstream
benthic habitats for mayfly larvae.
Rainbow trout captured at Site 4 consumed nearly 40% Ephemeropterans by
mass and their stomachs also contained a greater abundance of unidentified
insect remains. Additionally,
Ephemeroptera abundance greatest in Sites 3 and 4 and taken with diet trends,
this suggests the presence of an environmental gradient that favors benthic
Ephemeropterans as one moves downstream of the spillway. Conversely, isopods were only observed in
diets collected at Sites 1 and 2. This
could be due to close proximity to the reservoir where these food items are
being released directly from the reservoir.
Trout of all size classes primarily ate Gastropoda,
algae, and detritus even though the orders Diptera and Ephemeroptera were the
most abundant taxa among drift and benthic samples. These results are consistent with Halloran’s
finding (2000) because the most abundant drifting macroinvertebrate taxa from
the tailrace were disproportionately represented in trout diets. Results here are also consistent with Johnson
et al. (2006) where tail-water trout were eating a poor diet and a low
diversity of prey items. From Cummins
and Wuycheck (1971), Gastropods contain approximately 2,000 cal/g (dry
mass) and algae/detritus contain ca. 4,000 cal/g (dry mass). These values are lower than the averages for
aquatic insects (e.g. Ephemeroptera, Trichoptera, and Pyralidae) in the range of
5,000 to 6,000 cal/g (dry mass).
Ultimately, the apparent inordinate amount of Gastropoda consumed may be
an artifact of their recalcitrant structures that are also heavier by mass
relative to their abundance when compared to the exoskeleton of insects.
Because
all of the rainbow trout in this system were at one time stocked, their feeding
habits may also reflect their life histories.
Due to cold temperatures and relatively low food abundance, recently
stocked trout had a disadvantage heading into the winter months. Regardless of the system, recently stocked
trout may experience increased overwinter mortality for several reasons but
primarily food constraints and increased stress due to relocation (Simpkins and
Hubert 2000). It is possible that many
of the trout sampled were in good body condition simply because they were
sampled soon after they were stocked; not later in time when body condition
would deteriorate due to a poor diet as wild trout and stocked trout do
demonstrate different feeding habits in tailwater systems (Simpkins and Hubert
2000). While there are no naturally
reproducing rainbow trout, or wild trout in a true sense in the Guadalupe
River, there are trout that survive year-round.
It may be that some of the diet variability observed among sites is due
to acclimation of stocked trout to wild feed.
In a Wyoming tailwater, Simpkins and Hubert (2000) observed that stocked
trout tended to consume more benthic invertebrates where wild trout fed more
upon zooplankton (found in the drift).
This suggests that stocked rainbow trout, similar to the trout in this
study, tend to feed from the benthos rather than the drift which consists of a
more nutritious menu of prey items.
Consequently, the stocked trout in Simpkins and Hubert (2000) tended to
have a slightly lower mean of relative weight (Wr) than wild trout; significantly less in September, October and
November.
There
are at least three hypotheses that explain the paradox between the trout diets,
prey availability, and body condition. That is, these apparently healthy trout
were feeding upon food items that are both low in relative abundance and
caloric content. First, during the
summer months, these trout might be consuming terrestrial food items that could
subsidize their annual energy budgets.
Even during February, some terrestrial food items were detected. Second, these fishes might be consuming
zooplankton that was not picked up in the drift samples or the diets as they were
small and difficult to observe. Third,
it might also be the case where these fishes were recently stocked and the
deterioration of their body condition was not yet observed.
There
was very little terrestrial input observed in the trout diets in winter season. The only terrestrial items observed were formicids
and the group contributed a very small percentage of the overall diets (<
1.0%). The Allen Paradox (Allen 1952)
addresses the observation that autochthonous stream productivity is below that
required to sustain the observed trout biomass in certain streams. One explanation is that the additional energy
is derived from the adjacent terrestrial ecosystem in the form of terrestrial
arthropods. In fact, stream trout have
been reported to derive a majority of their annual energy budgets from
terrestrial food resources, especially during summer months in temperate
systems during certain times of year (Wipfli 1997; Nakano and Murakami 2001). Because there were very few terrestrial diet
inputs observed, the rainbow trout in the present study are not supplementing
their diets with terrestrial food items but it is also true that diets were
only sampled in February, a time of low terrestrial arthropod inputs in general. In other studies, both the availability and
the consumption of terrestrial invertebrates peak in the summer (Wipfli 1997;
Eberle and Stanford 2009). It could be
that Guadalupe River rainbow trout are in fact utilizing terrestrial food items
but only during warmer months not sampled.
ACKNOWLEDGMENTS
We would like to thank
the Guadalupe River Chapter of Trout Unlimited, Texas State River Systems
Institute, and the Texas State University Department of Biology for funding.
LITERATURE
CITED
Allen, K.R. 1951. The
Horokiwi stream: A study of a trout population.
New Zealand Department of Fisheries Bulletin 10.
Bettoli, P.W., S.J.
Owens, and M. Nemeth. 1999. Trout habitat, reproduction, survival, and growth
in the south fork of the Holston River. Fisheries Report Number 99-3, U.S.
Geological Survey, Tennessee Cooperative Fishery Research Unit, Cookeville, TN.
Bradle, T.A., Magnelia,
S.J. and J.B. Taylor. 2006. Trout angler utilization, attitudes, opinions, and
economic impact at the Canyon Reservoir tailrace. Texas Parks and Wildlife, Final
Report PWD RP T3200-1205, Austin.
Bunn, S.E. and
Arthington, A.H. 2002. Basic principles and ecological consequences of altered
flow regimes. Environmental Management 30:492-507.
Cada, G. F., J. M.
Loar, and M. J. Sale. 1987. Evidence of food limitation of rainbow and brown
trout in southern Appalachian soft-water streams. Transactions of the American
Fisheries Society 116:692-702.
Carlander, K.D. 1970. Handbook
of freshwater fishery biology. Vol 1. Pages 170-171. The Iowa State University
Press, Ames, Iowa, U.S.A.
Caudill, J. 2005. The economic
effects of rainbow trout stocking by fish and wildlife service hatcheries in FY
2004. U.S. Fish and Wildlife Service,
Division of Economics, Arlington, Virginia, December, 2005.
Chappman, D.W. 1966.
Food and space as regulators of salmonid populations in streams. American
Naturalist 100:345-357.
Cummins, K.W. and
Wuycheck, J.C. 1971. Caloric equivalents for investigations in ecological
energetics. Mitt. Internat. Verein. Limnol. 18.
Eberle, L.C., and
Stanford, J. 2009. Importance and seasonal availability of terrestrial
invertebrates as prey for juvenile salmonids in floodplain spring brooks of the
Kol River (Kamchatka, Russian Federation). River Research Applications. DOI:
10.1002/rra.
Elliott, J. M., 1973.
The food of brown and rainbow trout (Salmo
trutta and S. gairdneri) in relation
to the abundance of drifting invertebrates in a mountain stream. Oecologia
12:329-347.
Halloran, B.T.
2000. Foraging of introduced rainbow
trout Oncorhynchus Mykiss in relation to benthic macroinvertebrates and drift
in the Guadalupe River tailwater below Canyon reservoir, Texas. Master’s thesis,
Southwest Texas State, Texas.
Hannan, H.H., I.R.
Fuchs, and D.C. Whitenbert. 1979.
Spatial and temporal patterns of temperature, alkalinity, dissolved
oxygen and conductivity in an oligo-mesotrophic, deep-storage reservoir in
Central Texas. Hydrobiologia 66:209-221.
Jacobs, K.E., Swink,
W.D., and J.F. Novotny. 1987. Minimum tailwater flows in relation to habitat
suitability and sport-fish harvest. North American Journal of Fisheries
Management 7:569-574.
Johnson, R.L.,
Blumenshine, S.C., and S.M. Coghlan. 2006. A bioenergetics analysis of factors
limiting brown trout growth in an Ozark tailwater river. Environmental Biology
of Fishes 77:121-132.
Magnelia, S.J. 2004.
Summary of 1987-2001 data from the Canyon Reservoir Tailrace with implications
for establishment of a put-grow-and-take rainbow trout fishery. Management Data
Series 215. Texas Parks and Wildlife Department, Austin.
Mason,
J.C. 1976. Response of underyearling coho salmon to supplemental feeding in a
natural stream. Journal of Wildlife Management 40:775–788.
McCafferty,
W.P. 1998. Aquatic entomology: The fishermen’s and ecologists’ illustrated
Guide to Insects and
Their Relatives. Jones and Bartlett Publishers, Sudbury, MA.
Merritt, R.W. and K.W.
Cummins, editors. 1996. An Introduction To the Aquatic Insects of North
America. Kendall/Hunt Publishing Company, Dubuque, Iowa.
Murphy, M.L., Hawkings,
C.P., and N.H. Anderson. 1981. Effects of canopy modifications and accumulated
sediment on stream communities. Transactions of the American Fisheries Society
110:469-478.
Nakano, S. and
Murakami, M. 2001. Reciprocal subsidies: dynamic interdependence between
terrestrial and aquatic food webs. Proceedings of the National Academy of
Sciences 98: 166–170.
Nislow, K. H., C. Folt,
and M. Seandel. 1998. Food and foraging behavior in relation to microhabitat
use and survival of age-0 Atlantic salmon. Canadian Journal of Fisheries and
Aquatic Sciences 55:116-127.
Peckarsky, B.L., P.R.
Fraissinet, M.S. Penton, and D.J. Conklin Jr. 1990. Freshwater
Macroinvertebrates of Northeastern North America. Cornell University Press,
Ithaca, New York.
Poff, N. L., and A. D.
Huryn. 1998. Multi-scale determinants of secondary production in Atlantic
salmon (Salmo salar) streams. Canadian Journal of Fisheries and Aquatic
Sciences 55:201-217.
Richardson, J. S. 1993.
Limits to productivity in streams: evidence from studies of macroinvertebrates.
p. 9-15. In R.J. Gibson and R.E. Cutting (editors). Production of Juvenile
Atlantic salmon, Salmo salar, in natural waters. Canadian Special Publication
of Fisheries and Aquatic Sciences 118.
Simpkins, D.G. and
Hubert, W.A. 2000. Drifting invertebrates, stomach contents, and body
conditions of juvenile rainbow trout from fall through winter in a Wyoming
tailwater. Transactions of the American Fisheries Society 129:1187-1195.
Smith, D.G. 2001.
Pennak’s Freshwater Invertebrates of the United States: Porifera to Crustacea.
John Wiley and Sons, New York, New York.
Strauss, R.E. 1979.
Reliability estimates for Ivlev’s electivity index, the forage ratio, and
proposed linear index of food selection. Transactions of the American Fisheries
Society 108:344–352.
Weiland, M.A., and
Hayward, R.S. 1997. Cause for the decline of large rainbow trout in a tailwater
fishery: too much putting or too much taking? Transactions of the American
Fisheries Society 126:758-773.
White, R.L. 1968.
Evaluation of catchable rainbow trout fishery. Texas Parks and Wildlife
Department, Federal Aid in Sport Fish Restoration Project F-2-15, Job E-9,
Austin.
Wipfli, M.S., 1997.
Terrestrial invertebrates as salmonid prey and nitrogen sources in streams:
contrasting old-growth and young-growth riparian forests in southeastern
Alaska, U.S.A. Canadian Journal of Fisheries and Aquatic Sciences 54:1259–1269.
Zhang, Y.X. and
Richardson, J. S. 2007. Unidirectional prey-predator facilitation: apparent
prey enhance predator's foraging success on cryptic prey. Biology Letters 3:348-351
TABLES
Table 1. Diversity
calculations for diel drift samples taken in February 2007. Site 3 is the most diverse in terms of both
richness and evenness given the relatively high Shannon-Weiner and Simpson’s
values.
|
|
Site 1 |
Site 2 |
Site 3 |
|
Number of species |
22 |
18 |
25 |
|
Shannon Diversity |
1.5 |
1.2 |
1.9 |
|
Shannon Evenness |
0.49 |
0.41 |
0.58 |
|
Simpson Diversity |
0.54 |
0.44 |
0.71 |
Table 2. Chi-square
table to test whether or not rainbow trout are feeding on benthic organisms
commensurate with their relative abundances.
Because there were very few Ephemeropterans and Dipterans were uncommon
(or absent) in diet samples and the apparent selectivity for Gastropods, the
results is highly significant (χ² = 29.4, Df = 5, p < 0.001)
|
Taxa |
Observed Mass |
Expected Mass |
|
Ephemeroptera |
3.5 |
6.3 |
|
Diptera |
0.0 |
6.0 |
|
Amphipoda |
0.0 |
0.9 |
|
Gastropoda |
10.2 |
0.8 |
|
Coleoptera |
0.0 |
0.5 |
|
Hemiptera |
1.0 |
0.1 |
FIGURE
CAPTIONS
Figure 1. The study
sites used on the Guadalupe River below Canyon Reservoir in South-Central
Texas.
Figure 2. Annual
relative abundance of the most abundant benthic aquatic insects collected
within each season (summer = June, July, and August, fall = September, October,
and November, winter = December, January, and February, Spring = March, April,
and May). For each season, taxa that
contributed ≤ 2% relative abundance by number were removed.
Figure 3. Diel drift
net samples collected at Sites 1 – 3 during February 2007. Abundance on the y-axis refers to the total
number within each site at the time intervals on the x-axis.
Figure 4. Bi-plot of
the CCA for site ordination and benthic aquatic insect habitat
associations. The separation of sites
across the first axis is primarily due to mean depth and mean maximum
depth. Sites 1, 2, and 4 tended to be
deeper on average. The separation on the
second axis is mainly attributed to flow; Site 3 had higher velocities while
site 2 tended to have more moderate flows.
The associated aquatic invertebrate taxa separated from the main plot
were present in diet analyses.
Figure 5. Percent composition of trout diets
(wet mass, g) of prey items in rainbow trout stomachs by length class. Gastropoda and detritus make up a significant
amount of ingested material among all length classes but this represents a
relatively poor diet in terms of energy.
Figure 6. Rainbow trout
diets by site for the most abundant taxa (taxa contributing ≤ 2%
abundance by mass were removed).
Figure 7. Total length
vs. relative weight of rainbow trout sampled during February 2007 (n =
46). Dashed line indicates the 100 mark
on the y-axis, indicating an individual is of “quality” body condition for a
given length.
Figure 1. The study
sites were located on the Guadalupe River below Canyon Reservoir in
South-Central Texas.
Figure 2. Annual relative abundance of
the most abundant benthic aquatic insects collected within each season (summer
= June, July, and August, fall = September, October, and November, winter =
December, January, and February, Spring = March, April, and May). For each season, taxa that contributed
≤ 2% relative abundance by number were removed.
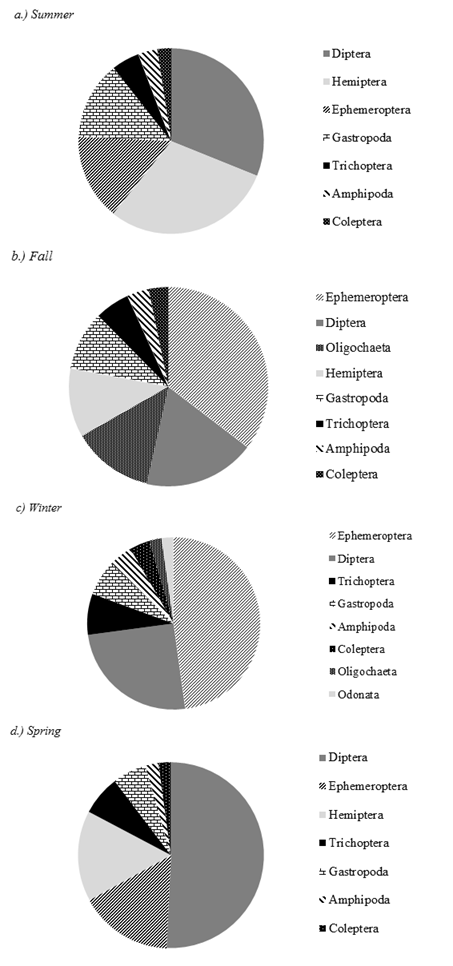
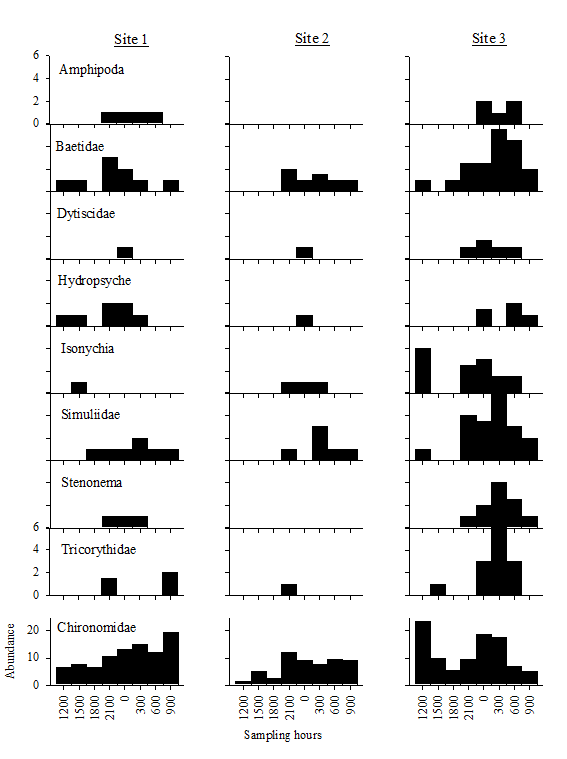
Figure 3.
Diel drift net samples collected at Sites 1–3 during February 2007. Abundance on the y-axis refers to the total
number within each site at the time intervals on the x-axis.
Figure 4. Bi-plot of the CCA for site
ordination and benthic aquatic insect habitat associations. The separation of sites across the first axis
is primarily due to mean depth and mean maximum depth. Sites 1, 2, and 4 tended to be deeper on
average. The separation on the second
axis is mainly attributed to flow; Site 3 had higher velocities while site 2
tended to have more moderate flows. The
associated aquatic invertebrate taxa separated from the main plot were present in
diet analyses.
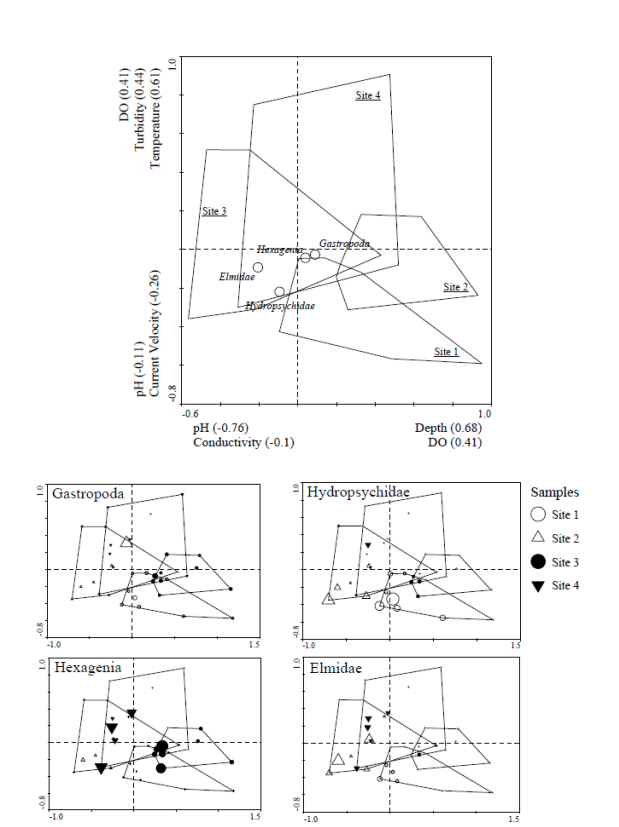
Figure 5. Percent composition of trout diets (wet
mass, g) of prey items in rainbow trout stomachs by length class. Gastropoda and detritus make up a significant
amount of ingested material among all length classes but this represents a
relatively poor diet in terms of energy.
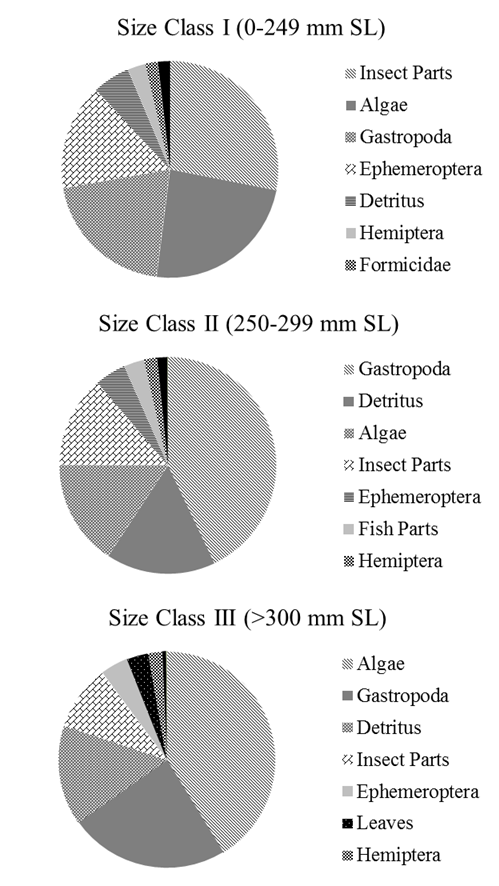
Figure 6. Rainbow trout diets by site for the most
abundant taxa (taxa contributing ≤ 2% 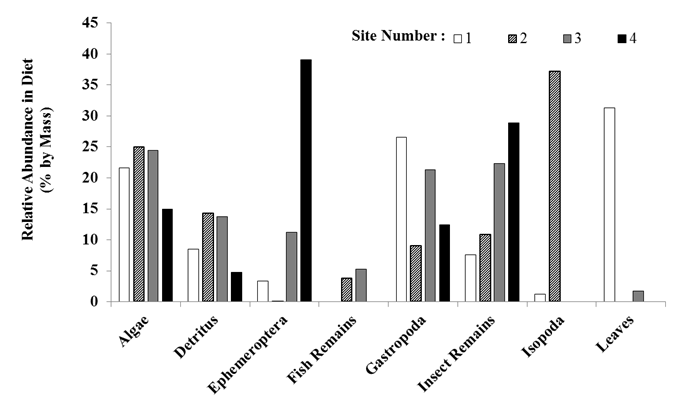 abundance by mass were removed).
abundance by mass were removed).
Figure 7. Total length vs. relative
weight of rainbow trout sampled during February 2007 (n = 46). Dashed line indicates the 100 mark on the
y-axis, indicating an individual is of “quality” body condition for a given
length.
Lay Fisher’s Glossary to Sullivan
et al.’s “Winter
Diet Composition of Introduced Rainbow Trout and Macroinvertebrate Assemblages
in the Guadalupe River, Texas”
Arthropod: bug
Assemblage:
all the different organisms that are found in a particular location
Autochthonous:
energy produced in a stream’s ecosystem (aquatic plants or algae)
Baetidae/Baetids: family of
mayfly



Benthic:
relating to the bottom of a body of water
Bioenergetics:
the study of energy use and requirements in living organisms
Chironomidae/Chironomids: non-biting
midges


Conductivity:
amount of dissolved ions in the water
D-net:
hand-held net used to collect benthic macroinvertebrates

Detritus:
dead organic material
Diel:
over a 24-hour period
Diptera/Dipterans: members of the
fly family (mosquitoes, midges, crane flies, black
flies,
etc.)
Discharge:
the volume of water passing a given point in a river
Drift net: a cone-shaped
net set in a river to capture macroinvertebrates drifting in the
water column

Dytiscidae/Dytiscids: predaceous diving beetles

Elmidae: riffle beetles

Energy budgets: how many
calories a fish needs to consume for various life stage
requirements
(maintenance, growth, reproduction) vs. how many calories they
actually
consume
Ephemeroptera/Ephemeropterans: mayflies


Formicidae: ants


Gastropoda/Gastropods: snails


Hemiptera/Hemipterans: members of the
true bug family (water striders, water scorpions,
giant water
bugs, water boatmen, etc.)



Hexagenia: genus of large, yellow mayflies


Hydropsychidae/Hydropsychid: net-spinning
caddis flies



Hypolimnion: the bottom
part of a lake, typically colder and lower in dissolved oxygen
than the upper layers.
Isonychia: genus of mayfly


Isopods/Isopoda: scuds


Lotic:
moving waters/rivers
Lowest practical resolution/lowest
practical taxonomic level: as specific as practically
possible
with regard to taxonomic classification, usually to the genus level
Macroinvertebrate:
bugs large enough to see with the naked eye
Mesohabitats: basic
structural elements of a river or stream such as pools, backwaters,
runs, glides, and riffles
Multivariate direct gradient ordination technique
(canonical correspondence analysis;
CCA): statistical
method of associating sites and organisms along a gradient of
environmental parameters
Naucoridae: predaceous
water bugs

Odonata/Odonates: damselflies
and dragonflies




Oligochaeta: segmented
worms


Pyralidae/Pyralids: family of
moths with aquatic larvae


Recalcitrant:
hard to digest/break down
Riffle: shallow, fast-moving water

Run: smooth-flowing, deeper water
with moderate speed

Salmonids:
fish belonging to the salmon family (trout, char, and salmon)
Shannon-Wiener (H) and Simpson’s (D)
diversity indices:
different measures of the
diversity
of living organisms in a collected sample
Simuliidae/Simuliid: blackflies



Stenonema: genus of mayfly


Substrate:
material on the bottom of the river
Surber sample: a method of
collecting benthic macroinvertebrates in flowing water


Taxa:
taxonomic group (i.e. family, genus, species, etc.)
Trichoptera/Trichopterans: caddis flies



Tricorythidae: fwamily of small mayflies (Tricos)



Turbidity:
clarity of the water (more turbid = less